Empower your business with BioFire panels. From faster detection to better patient management, these tests help you deliver accurate, timely results – boosting trust, outcomes and your business potential.
Respiratory Panel
22 Pathogens Detected
This test uses Multiplex PCR from nasopharyngeal swab specimens. It enables rapid simultaneous detection of numerous viral and bacterial pathogens associated with upper respiratory tract infections.
This assay identifies specific nucleic acid sequences. It facilitates faster and more accurate differential diagnosis and treatment decisions.
One Patient | 22 Target In One Comprehensive Test | Improves Patient Management
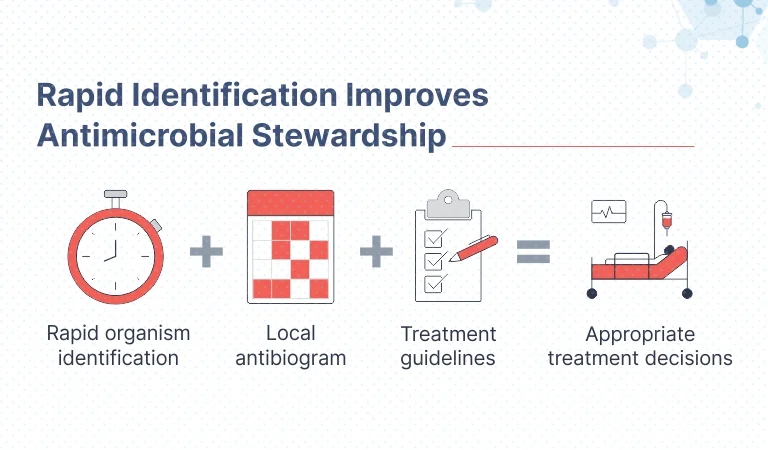
Superior Clinical & Economic Outcomes
Studies show that the BioFire RP Panel has been shown to:
- Dramatically Reduce time to diagnosis
- Improve patient management
- Reduce unnecessary testing
- Reduce total cost of care and resource utilization
- Prevent exposure to unnecessary antibiotics
- Prevent secondary spread of infection
- Result in shorter hospital stays
- Provide more timely and effective treatment
- Detects more positives & co-infections than non-panel assays.
Biofire® Respiratory 2.1 (RP2.1) Panel
Sample type: Nasopharyngeal swab in transport media
Test code: BIORP
Processed at: CPL
TAT: 24 Hours
Pathogens Covered
Viruses:
- Adenovirus
- Coronavirus 229E
- Coronavirus HKU1
- Coronavirus NL63
- Coronavirus OC43
- Severe Acute Respiratory Coronavirus 2 (SARS-CoV-2)
- Human Metapneumovirus
- Human Rhinovirus/Enterovirus
- Influenza A
- Influenza A/H1
- Influenza A/H1 – 2009
- Influenza A/H3
- Influenza B
- Parainfluenza Virus 1
- Parainfluenza Virus 2
- Parainfluenza Virus 3
- Parainfluenza Virus 4
- Respiratory Syncytial Virus
Bacteria:
- Bordetella parapertussis
- Bordetella pertussis
- Chlamydia pneumoniae
- Mycoplasma pneumoniae
BioFire RP2.1 Panel Performance
Overall
- 97.4% Sensitivity
- 99.4% Specificity
Go To Market
- ICU beds in 20-50 bedded hospitals & Critical Care Units
- Intensivist, Pediatric – NICU
- Immunocompromised patients
(Transplant patients, Oncology patients etc.)
- Pulmonologists
Blood Culture® Identification Panel
43 Targets
The BioFire Blood Culture Identification (BCID) Panel is a multiplex PCR- based assay designed for the rapid and simultaneous detection of a broad spectrum of bacterial, fungal and antimicrobial resistance genes directly from positive blood culture samples.
This test facilitates the expedited identification of causative pathogens in bloodstream infections (BSIs), including sepsis, a life-threatening condition characterized by dysregulated host response to infection.

Who Should Get Tested?
Positive blood cultures from adult and pediatric patients with monomicrobial or polymicrobial bloodstream infections.
- Children and adults
- Elderly Patients
- High risk patients: immuno-compromised or with co-morbidities
- Critically ill patients
Timely & Accurate diagnosis leads to better BSI outcomes:
- Lessens unnecessary antibiotic use
- Improves time to anti-microbial de-escalation
- Decreases time to effective therapy
- Reduces hospital costs, including by reducing repeat blood cultures & length of stay
Biofire® Blood Culture Identification 2 (BCID2) Panel
Sample Type: Positive Blood Culture Sample
Test Code: BIOBC
Processed At: CPL
TAT: 24 Hours
Pathogens covered
GRAM-NEGATIVE BACTERIA
- Acinetobacter calcoaceticusbaumannii complex
- Bacteroides fragilis
- Enterobacterals:
- Enterobacter cloacae complex
- Escherichia coli
- Klebsiella aerogenes
- Klebsiella oxytoca
- Klebsiella pneumoniae group
- Proteus spp.
- Salmonella spp.
- Serratia marcescens
- Haemophilus influenzae
- Neisseria meningitidis
- Pseudomonas aeruginosa
- Stenotrophomonas maltophilia
GRAM – POSITIVE BACTERIA
- Enterococcus faecalis
- Enterococcus faecium
- Listeria monocytogenes
- Staphylococcus spp.
- Staphylococcus aureus
- Staphylococcus epidermidis
- Staphylococcus lugdunensis
- Streptococcus spp.
- Streptococcus agalactiae
- Streptococcus pneumoniae
- Streptococcus pyogenes
YEAST
- Candida albicans
- Candida auris
- Candida glabrata
- Candida krusei
- Candida parapsilosis
- Candida tropicalis
- Cryptococcus
(C. neoformans/ C. gattii)
ANTIMICROBIAL RESISTANCE GENES
Carbapenemases
- IMP
- KPC
- OXA-48-like
- NDM
- VIM
Colistin Resistance
- mcr-1
ESBL
- CTX-M
Methicillin Resistance
- mecA/C
- mecA/C and MREJ (MRSA)
Vancomycin Resistance
- vanA/B
Go To Market
- ICU beds in 20-50 bedded hospitals & Critical Care Units
- Intensivist, Pediatric – NICU
- Immunocompromised patients
- (Transplant patients, Oncology patients etc.)
- Pulmonologists,
- MD Medicine Doctors
PNEUMONIA PANEL
34 Targets
The BioFire Pneumonia Panel uses multiplex PCR to detect a comprehensive array of bacterial, viral and fungal pathogens directly from lower respiratory tract specimens, including sputum, bronchoalveolar lavage (BAL) and endotracheal aspirates.
This assay facilitates rapid identification of causative agents in pneumonia, enabling targeted microbial therapy and improved clinical management. It enhances diagnostic accuracy and reduces time to pathogens identification compared to traditional culture-based methods.
Significance
Pneumonia patients require appropriate therapy quickly.
Challenges in Pneumonia:
- Traditional culture methods are insensitive & time consuming
- Initial antibiotic treatment fails in 1 to 5 community-acquired pneumonia patients, requiring an additional or different antibiotic course, an ER Visit or hospitalization.
The right test, the first time:
- The BioFire FilmArray Pneumonia Panel plus gives sensitive, accurate results from a variety of commonly collected lower respiratory tract specimens. These fast, reliable results may allow clinicians to have confidence in making targeted therapy decisions.
- Detects more bacteria: PCR detects nucleic acids rather than only organisms that will grow on culture media, detecting many more bacteria than culture.
- Reports semi-quantative results: Provides relative abundance information for 15 bacteria that can be a pathogen or normal flora.
- It improves patient care by improving time to targeted therapy, reducing treatment cost and improving laboratory and patient management workflows.
- It helps in Appropriate Antimicrobial Stewardship.
Biofire-Pneumonia Plus Panel
Sample Type: Bronchoaleveolar Lavage (BAL), Sputum, Endotracheal secretion (ETA)
Test Code: BIOPP
Processed At: CPL
TAT: 24 Hours
Pathogens Covered
Bacteria:
- Acinetobacter calcoaceticus baumannii complex
- Enterobacter cloacae complex
- Escherichia coli
- Haemophilus influenzae
- Klebsiella aerogenes
- Klebsiella oxytoca
- Klebsiella pneumoniae group
- Moraxella catarrhalis
- Proteus spp.
- Pseudomonas aeruginosa
- Serratia marcescens
- Staphylococcus aureus
- Streptococcus agalactiae
- Streptococcus pneumoniae
- Streptococcus pyogenes
Atypical Bacteria:
- Chlamydia pneumoniae
- Legionella pneumophila
- Mycoplasma pneumoniae
Viruses:
- Adenovirus
- Coronavirus
- Human Metapneumovirus
- Human Rhinovirus/ Enterovirus
- Influenza A
- Influenza B
- Middle East Respiratory Syndrome Coronavirus
- Parainfluenza Virus
- Respiratory Syncytial Virus
Antimicrobial Resistance Genes:
- Methicillin Resistance
mecA/C and MREJ
Carbapenemases
- IMP
- KPC
- NDM
- Oxa-48-like
- VIM
ESBL
- CRX-M
Diseases Under Surveillance:
- Influenza-including influenza A (H1N1)
- Legionnaires’ disease
- Haemophilus influenzae
Go To Market
- ICU beds in 20-50 bedded hospitals & critical care units
- Intensivist, Pediatric – NICU
- Immunocompromised patients
- (Transplant patients, Oncology patients etc.)
- Pulmonologists,
- Patients undergoing Incubation/ Ventilation / Bronchioscopy
Download Handouts Here:
Blood Culture: https://lead.thyrocare.com/wp-content/uploads/2025/07/Blood-Culture.pdf
Pneumonia: https://lead.thyrocare.com/wp-content/uploads/2025/07/PNEUMONIA.pdf
Respiratory Panel: https://lead.thyrocare.com/wp-content/uploads/2025/07/Respiratory-Panel-1.pdf
Combined Handout: https://lead.thyrocare.com/wp-content/uploads/2025/07/Biofire-Booklet_ctcv-2.pdf
We hope you found this article helpful! Keep exploring Knowledge Base for the latest updates on tests, offers and more.